
New Anti-Viral Treatment Hacks the Virus’ Protective Shield
-
by Anoop Singh
- 36
Lenacapavir, an innovative anti-retroviral drug, thwarts HIV replication by destabilizing its protective capsid, with research from UNSW Sydney providing crucial insights into its mechanism and potential for further antiviral developments.
Just over a year ago, the European Union and the US Food and Drug Administration approved a new anti-retroviral medication aimed at combating human immunodeficiency virus (HIV) infections. Named Lenacapavir, this medication represents the first of its kind to specifically target the protective shell of the HIV virus, known as the HIV capsid, offering a new approach to treatment for patients.
An international team of researchers led by UNSW Sydney medical researchers now have the details on how this novel drug pushes the HIV capsid to breaking point, stopping the virus in its tracks. The molecular mechanisms that they uncovered are published in the journal eLife, and could help to refine and design more effective anti-viral therapies.
HIV encases its genetic material in a protein coat to safeguard the virus as it converts its genomic RNA into DNA enroute to the nucleus after entering the target cell. Developed by biopharmaceutical company Giliad Sciences, lenacapavir was designed to thwart this very protection afforded by the capsid. And this potent and long-acting drug is the first, and so far the only, approved anti-HIV therapy to do so.
“The fact that the capsid plays a central role in multiple stages of the viral life cycle, and therefore represents a really good drug target, is a concept that’s only emerged in recent years,” said Professor Till Böcking, who led the team together with Dr David Jacques.
Building them tough to break them down
By combining cell infection studies with single-molecule imaging, the researchers showed how lenacapavir disrupted the HIV life cycle. Some theorized that the drug hardens the capsid to lock the virus in, thereby preventing it from establishing infection. Instead, the team saw that the capsid, fortified by the drug, actually became very brittle.
“What we found was that this hyper-stabilization actually led to a premature breakage of the capsid, before the virus can finish converting its RNA into DNA,” Professor Böcking said.
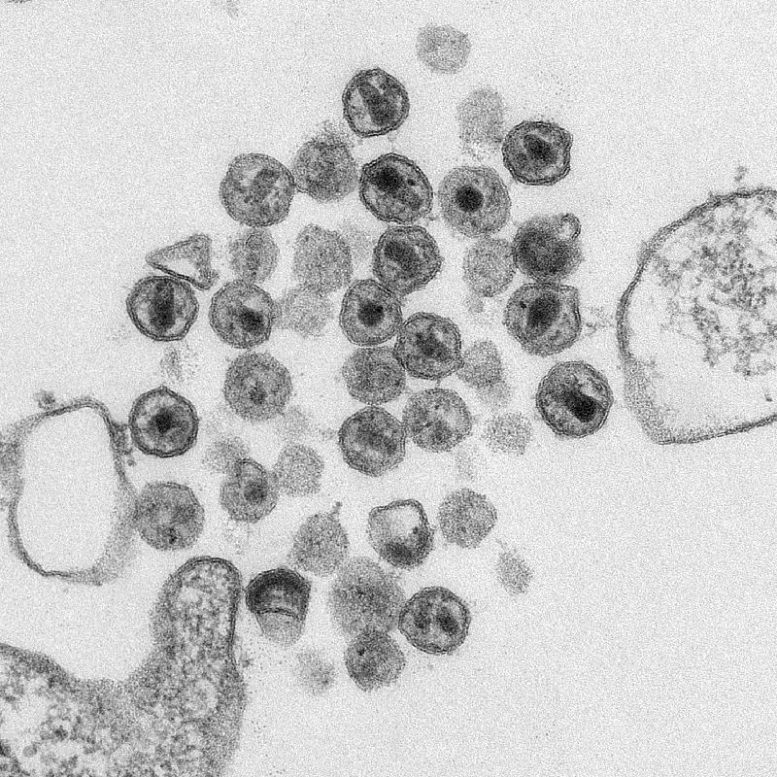
Lenacapavir causes the HIV capsid to rupture before it can transport its genetic material to the host cell nucleus. Credit: Public Health Image Library, CDC
In the target cell, the capsid would rupture before the virus reaches the nucleus, leaving its genetic material exposed to the hostile environment in the host cell cytoplasm. To study the effect of lenacapavir on individual capsids over time, the team worked with non-infectious HIV-like particles produced by cells.
“With our microscope setup, we can look at the integrity of the capsids. By monitoring the release of fluorescent tags loaded into the capsid, we can work out exactly when it cracks,” said Dr Walsh, one of the study’s lead authors.
With Dr. Leo James and other colleagues in the Laboratory of Molecular Biology in the UK, the team also examined the building of new capsids, recreating a process that would take place after newly made copies of the viral genome are bundled up for release from infected cells. They found that lenacapavir sabotaged capsid integrity at this phase of the HIV life cycle as well by accelerating the capsid construction to force construction errors. The deformed capsids that were produced were unable to close properly and would fail to shield the viral genome from attack.
This study not only settles the debate over whether capsid-targeting drugs strengthen or weaken the capsid, the uncovered mechanism could also be exploited for targeting other viruses that build capsids to shelter from host defenses.
“Lenacapavir is orders of magnitude better than any other compound that targets the capsid. Our results give a really good blueprint for how this drug is able to be so incredibly effective,” said Dr Walsh.
Reference: “Pharmacologic hyperstabilisation of the HIV-1 capsid lattice induces capsid failure” by KM Rifat Faysal, James C Walsh, Nadine Renner, Chantal L Márquez, Vaibhav B Shah, Andrew J Tuckwell, Michelle P Christie, Michael W Parker, Stuart G Turville, Greg J Towers, Leo C James, David A Jacques and Till Böcking, 13 February 2024, eLife.
DOI: doi:10.7554/eLife.83605
The study was funded by the National Health and Medical Research Council, the Wellcome Trust, and Australian Research Council.
Lenacapavir, an innovative anti-retroviral drug, thwarts HIV replication by destabilizing its protective capsid, with research from UNSW Sydney providing crucial insights into its mechanism and potential for further antiviral developments. Just over a year ago, the European Union and the US Food and Drug Administration approved a new anti-retroviral medication aimed at combating human immunodeficiency…
Lenacapavir, an innovative anti-retroviral drug, thwarts HIV replication by destabilizing its protective capsid, with research from UNSW Sydney providing crucial insights into its mechanism and potential for further antiviral developments. Just over a year ago, the European Union and the US Food and Drug Administration approved a new anti-retroviral medication aimed at combating human immunodeficiency…
