
Immune Cells Carry a Long-Lasting “Memory” of Early-Life Pain
-
by Anoop Singh
- 9
New research indicates that pain experienced by newborns can lead to long-lasting genetic changes in their immune cells, intensifying pain responses as they grow, especially in females, highlighting the need for more specific treatments. Credit: SciTechDaily.com
Research in mice indicates that focusing on genetic alterations in macrophage cells could be beneficial.
Recent research increasingly suggests that the human body can retain memories of pain from injuries sustained in infancy, including life-saving surgeries, well into adolescence.
These early experiences appear to change how a child’s pain response system develops at a genetic level, resulting in more intense reactions to pain later in life. Such changes also appear to occur more often among females.
Now research led by experts at Cincinnati Children’s pinpoints how and where the genetic changes that create such long-lasting pain memory occur. According to their study, published in the journal Cell Reports, the key changes are occurring in developing macrophage cells—one of the major elements of the immune system.
“Our experiments help to further confirm how pain memories affect female newborns for longer periods of time. Specifically, our data indicates that an epigenetic change (changes that occur post-birth vs inherited gene variations) occurs in macrophages after an early-life injury, which in turn promotes more-intense pain responses to other injuries that occur later in life,” says corresponding author Michael Jankowski, PhD, associate director of the Pediatric Pain Research Center at Cincinnati Children’s.
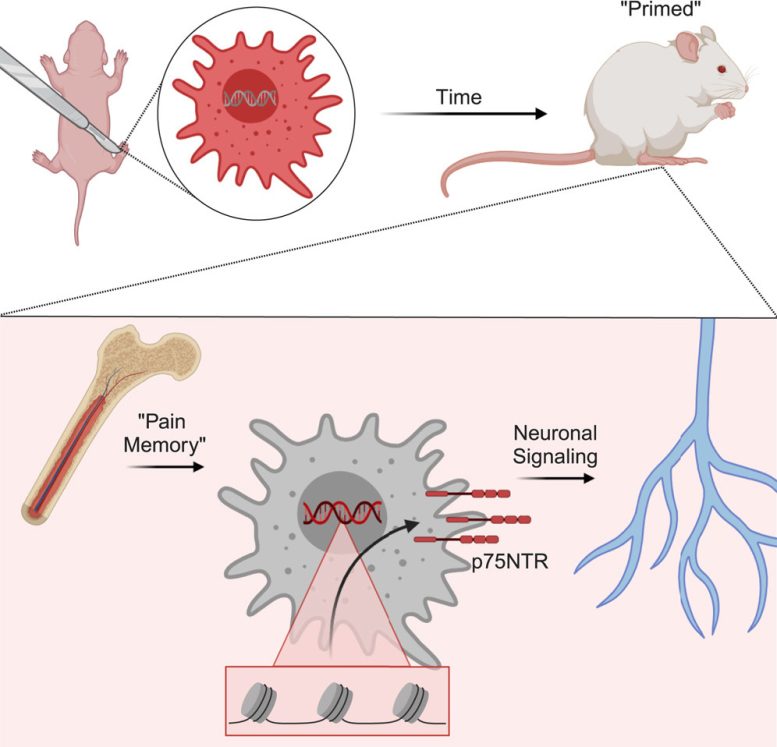
Early-life injury can change how the body’s pain response system develops at a genetic level, leading to a pain “memory” that can affect response to injuries occurring years later, according to a study in Cell Reports published by experts at Cincinnati Children’s. Credit: Cell Reports and Cincinnati Children’s
Adam Dourson, PhD, now working at Washington University in St. Louis, was the study’s lead author.
The experiments show that male mice experiencing similar early-life injury show the same epigenetic changes but did not sustain the same long-term pain memory as females. Further testing also showed that changes, occurring in a gene called p75NTR, can be found in human macrophage cells.
In female mice, the pain memory effects were detected for more than 100 days after the initial injury. Incisions caused stem cells in the bone marrow to generate macrophages that were “primed” to respond more intensely to injuries, which in turn increases pain.
In humans, a similar timeframe would be roughly 10-15 years.
“It was surprising to us to see how a single, local insult so dramatically altered the systemic macrophage epigenetic/transcriptomic landscape,” Jankowski says.
This new understanding of neonatal pain memory underscores fundamental differences that exist between the genetic activity of a still-developing newborn immune system versus the mature system adults have. That means it will be complicated to determine how surgeons and care teams might go about adjusting how they manage recovery care for newborn and infant girls.
“Simply changing pain medication doses may not be the answer. There’s always a balancing act between controlling pain and minimizing the possible harmful side effects of existing medications. Instead, our findings suggest there’s a need to develop more-specific, better-targeted treatments that could prevent the re-programming of macrophage cells in response to injury,” Jankowski says.
Next steps
More research is needed to use this new information to develop therapies to control immune “pain memories.”
In this study, blocking the p75NTR receptor in young mice did blunt the ability of macrophages to communicate to sensory neurons and partially prevented prolonged pain-like behaviors. However, it remains unclear if similar methods can be safely used to target human macrophages.
“Emerging technologies appear capable of specifically blocking the p75NTR receptor in macrophages, but it will require considerably more research before this approach would be ready for human clinical trials,” Jankowski says.
Reference: “Macrophage memories of early-life injury drive neonatal nociceptive priming” by Adam J. Dourson, Adewale O. Fadaka, Anna M. Warshak, Aditi Paranjpe, Benjamin Weinhaus, Luis F. Queme, Megan C. Hofmann, Heather M. Evans, Omer A. Donmez, Carmy Forney, Matthew T. Weirauch, Leah C. Kottyan, Daniel Lucas, George S. Deepe and Michael P. Jankowski, 18 April 2024, Cell Reports.
DOI: 10.1016/j.celrep.2024.114129
Funding for this study included multiple grants from the National Institutes of Health (R01NS105715, R01NS113965, F31NS122494, R01HL160614, P30 AR070549; an ARC award from Cincinnati Children’s and support from the Leukemia and Lymphoma Society.
New research indicates that pain experienced by newborns can lead to long-lasting genetic changes in their immune cells, intensifying pain responses as they grow, especially in females, highlighting the need for more specific treatments. Credit: SciTechDaily.com Research in mice indicates that focusing on genetic alterations in macrophage cells could be beneficial. Recent research increasingly suggests…
New research indicates that pain experienced by newborns can lead to long-lasting genetic changes in their immune cells, intensifying pain responses as they grow, especially in females, highlighting the need for more specific treatments. Credit: SciTechDaily.com Research in mice indicates that focusing on genetic alterations in macrophage cells could be beneficial. Recent research increasingly suggests…
