Science Simplified: What Is a Battery?
-
by Anoop Singh
- 7
Batteries, from disposable AA to rechargeable lithium-ion types, are essential in converting chemical energy into electrical energy, with lithium-ion variants powering modern devices through a cyclical electron flow. Argonne National Laboratory plays a pivotal role in advancing battery technology, from fundamental research to recycling innovations.
What Is a Battery?
Batteries power our lives by transforming energy from one type to another.
Whether a traditional disposable battery (e.g., AA) or a rechargeable lithium-ion battery (used in cell phones, laptops, and cars), a battery stores chemical energy and releases electrical energy.
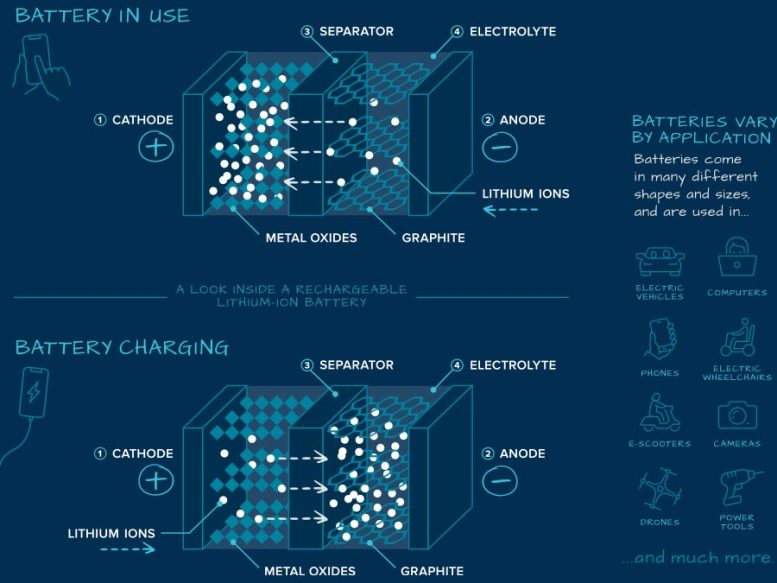
There are four key parts in a battery — the cathode (positive side of the battery), the anode (negative side of the battery), a separator that prevents contact between the cathode and anode, and a chemical solution known as an electrolyte that allows the flow of electrical charge between the cathode and anode.
In this Science 101: How Does a Battery Work? video, scientist Lei Cheng explains how the electrochemistry inside of batteries powers our daily lives. Whether a traditional disposable battery (e.g., AA) or a rechargeable lithium-ion battery (used in cell phones, laptops and cars), a battery stores chemical energy and releases electrical energy. Cheng mentions her research interests which are focused on batteries for electric vehicles and for the electric grid. For the latter, the goal is to use large and inexpensive batteries to store renewable energy (energy that comes from natural sources like the sun and wind) for use on the electric grid when the sun isn’t shining or the wind isn’t blowing.
Lithium-ion batteries that power cell phones, for example, typically consist of a cathode made of cobalt, manganese, and nickel oxides and an anode made out of graphite, the same material found in many pencils. The cathode and anode store the lithium.
When a lithium-ion battery is turned on, positively charged particles of lithium (ions) move through the electrolyte from the anode to the cathode. Chemical reactions occur that generate electrons and convert stored chemical energy in the battery to electrical current.
When you plug in your cell phone to charge the lithium-ion battery, the chemical reactions go in reverse: the lithium ions move back from the cathode to the anode.
As long as lithium ions shuttle back and forth between the anode and cathode, there is a constant flow of electrons. This provides the energy to keep your devices running. Since this cycle can be repeated hundreds of times, this type of battery is rechargeable.
Batteries and the U.S. Department of Energy’s (DOE) Argonne National Laboratory
Argonne is recognized as a global leader in battery science and technology. Over the past sixty years, the lab’s pivotal discoveries have strengthened the U.S. battery manufacturing industry, aided the transition of the U.S. automotive fleet toward plug-in hybrid and electric vehicles, and enabled greater use of renewable energy, such as wind and solar power.
The lab’s research spans every aspect of battery development, from the breakthrough fundamental science of the Argonne-led Joint Center for Energy Storage Research, a DOE Energy Innovation Hub, to the Argonne Collaborative Center for Energy Storage Science, a cross-lab collective of scientists and engineers that solves complex battery problems through multidisciplinary research.
Argonne researchers are also exploring how to accelerate the recycling of lithium-ion batteries through the DOE’s ReCell Center, a collaboration led by Argonne that includes the National Renewable Energy Laboratory, Oak Ridge National Laboratory, as well as Worcester Polytechnic Institute, University of California at San Diego and Michigan Technological University.
How does a lithium-ion battery work?
Lithium-based batteries power our daily lives, from consumer electronics to national defense.
A lithium-ion battery is a type of rechargeable battery. It has four key parts:
- The cathode (the positive side), typically a combination of nickel, manganese, and cobalt oxides
- The anode (the negative side), commonly made out of graphite, the same material found in many pencils
- A separator that prevents contact between the anode and cathode
- A chemical solution known as an electrolyte that moves lithium ions between the cathode and anode. The anode and cathode store lithium.
When the battery is in use, positively charged particles of lithium (ions) move through the electrolyte from the anode to cathode. Chemical reactions occur that generate electrons and convert stored chemical energy in the battery to electrical current.
When the battery is charging, the chemical reactions go in reverse: the lithium ions move back from the cathode to the anode.
Credit: Argonne National Laboratory
How Does a Lithium-Ion Battery Work?
Lithium-based batteries power our daily lives, from consumer electronics to national defense.
A lithium-ion battery is a type of rechargeable battery. It has four key parts:
- The cathode (the positive side), typically a combination of nickel, manganese, and cobalt oxides
- The How Does a Lithium-Ion Battery Work? (the negative side), commonly made out of graphite, the same material found in many pencils
- A How Does a Lithium-Ion Battery Work? that prevents contact between the anode and cathode
- A chemical solution known as an How Does a Lithium-Ion Battery Work? that moves lithium ions between the cathode and anode. The anode and cathode store lithium.
When the battery is in use, positively charged particles of lithium (ions) move through the electrolyte from the anode to cathode. Chemical reactions occur that generate electrons and convert stored chemical energy in the battery to electrical current.
When the battery is charging, the chemical reactions go in reverse: the lithium ions move back from the cathode to the anode.
Batteries, from disposable AA to rechargeable lithium-ion types, are essential in converting chemical energy into electrical energy, with lithium-ion variants powering modern devices through a cyclical electron flow. Argonne National Laboratory plays a pivotal role in advancing battery technology, from fundamental research to recycling innovations. What Is a Battery? Batteries power our lives by transforming…
Batteries, from disposable AA to rechargeable lithium-ion types, are essential in converting chemical energy into electrical energy, with lithium-ion variants powering modern devices through a cyclical electron flow. Argonne National Laboratory plays a pivotal role in advancing battery technology, from fundamental research to recycling innovations. What Is a Battery? Batteries power our lives by transforming…
